This tool helps identify potential gastrointestinal conditions based on symptoms. Note: This is not a diagnostic tool. Always consult a healthcare professional for proper diagnosis.
Key insight: Diabetic gastroparesis often shares symptoms with other GI disorders, but certain combinations are more indicative of specific conditions. Symptom overlap can lead to misdiagnosis without proper testing.
| Symptom | Diabetic Gastroparesis | GERD | IBS | Functional Dyspepsia |
|---|---|---|---|---|
| Early satiety | ✓ | ✗ | ✗ | ✓ |
| Bloating | ✓ | ✓ | ✓ | ✓ |
| Heartburn | ✗ | ✓ | ✗ | ✗ |
| Nausea/Vomiting | ✓ | ✓ (occasional) | ✓ (sometimes) | ✓ |
| Constipation/Diarrhea | ✓ | ✗ | ✓ | ✗ |
When diabetes slows the stomach’s rhythm, diabetic gastroparesis is a chronic condition where the stomach empties food too slowly. This slowdown isn’t because of a blockage; it’s a nerve‑related motility problem that can make everyday meals feel like a marathon. Understanding diabetic gastroparesis helps you see why it often shows up alongside other gastrointestinal disorders.
The term breaks down into three parts:
Key attributes of diabetic gastroparesis include:
Many GI conditions share bloating, nausea, and early satiety. Below is a quick snapshot of the overlap.
| Symptom | Diabetic Gastroparesis | GERD | IBS | Functional Dyspepsia |
|---|---|---|---|---|
| Early satiety | ✓ | ✗ | ✗ | ✓ |
| Bloating | ✓ | ✓ | ✓ | ✓ |
| Heartburn | ✗ | ✓ | ✗ | ✗ |
| Nausea/Vomiting | ✓ | ✓ (occasionally) | ✓ (sometimes) | ✓ |
| Constipation/Diarrhea | ✓ (often constipation) | ✗ | ✓ | ✗ |
Because of this overlap, clinicians often start with a broad GI work‑up before pinpointing gastroparesis.
Autonomic neuropathy is the technical name for nerve damage that controls involuntary organs. In diabetes, chronic hyperglycemia triggers oxidative stress, leading to:
Studies from 2023‑2024 show that patients with a HbA1c>9% have a 2.8‑fold higher risk of developing gastroparesis compared with those maintaining HbA1c<7%.
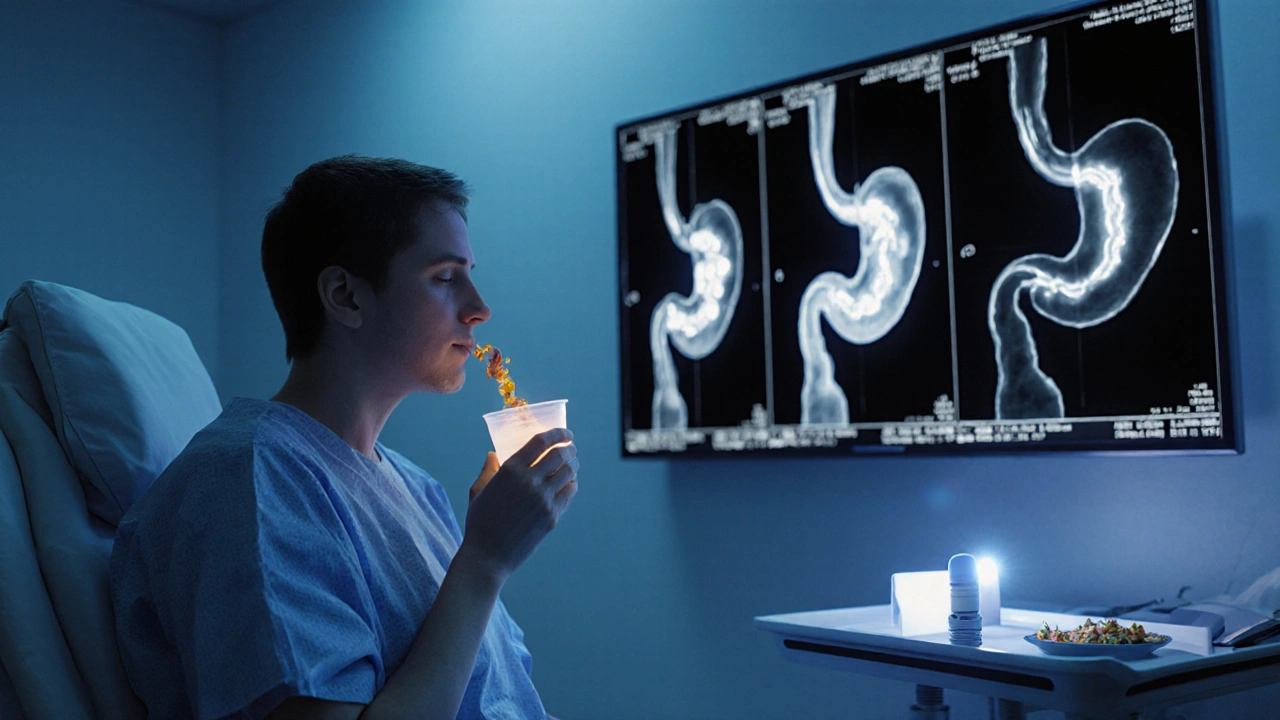
The gold standard remains gastric emptying scintigraphy (GES). The test involves swallowing a radiolabeled meal and taking images over four hours. A retention of >60% at two hours or >10% at four hours confirms delayed emptying.
Newer tools are emerging:
Regardless of the method, clinicians also rule out mechanical obstruction with endoscopy or CT imaging before labeling it gastroparesis.
Effective management hinges on two fronts: improving gastric emptying and tightening blood‑sugar control.
All pharmacologic choices should be paired with a diabetes care plan aiming for HbA1c<7% to slow neuropathy progression.

If a patient reports persistent heartburn despite proton‑pump inhibitor (PPI) therapy, or alternating constipation and diarrhea, clinicians should evaluate for GERD or IBS‑conditions that can coexist with gastroparesis. A practical rule of thumb:
Running a concurrent stool‑calorie test, pH monitoring, or a breath hydrogen test can pinpoint the secondary disorder.
Regular gastroparesis can result from surgery, medication, or neurological disease. Diabetic gastroparesis specifically stems from autonomic neuropathy caused by chronic high blood‑sugar levels.
Fiber slows gastric emptying, so high‑fiber foods are usually limited. A low‑fiber diet (1‑2g per meal) is recommended during active symptoms, but soluble fiber in small amounts may be tolerated.
In the UK, the NHS may fund GES for severe, refractory cases after multidisciplinary review, but private insurers vary widely.
A four‑hour gastric emptying scintigraphy is the quickest, standardized test. Breath‑test alternatives take about 2hours but are less widely available.
Tight control can halt progression and may improve symptoms, but existing nerve damage is often irreversible. Early intervention yields the best outcomes.
Got a quick takeaway: diabetic gastroparesis isn’t just about slow stomach emptying, it’s a whole autonomic nightmare. The vagus nerve gets fried by high glucose, and that messes with the whole motility cascade. What’s useful is the symptom overlap table – it shows why patients end up being shuffled between GERD, IBS, and dyspepsia clinics. If you’re looking at early satiety plus bloating, flag gastroparesis high on the differential. And remember, a gastric emptying study is the gold‑standard before you label it.
One might argue that the post oversimplifies the diagnostic algorithm; in reality, clinicians rely on a battery of tests beyond sheer symptom checklists. While the table is aesthetically pleasing, it neglects the nuanced pathophysiology that distinguishes dyspepsia from true gastroparesis. Moreover, the discussion of prokinetics skimps over the FDA’s stringent risk–benefit analysis.
Let me paint a picture, dear reader, for the stakes are nothing short of culinary apocalypse for those afflicted. Imagine trying to eat a simple slice of toast, only to feel as if you swallowed a brick; the stomach, once a diligent conveyor, has become a stagnant swamp of unprocessed morsels. This isn’t merely a hiccup in digestion – it’s a full‑blown insurgency by hyperglycemia against the vagus nerve, the maestro of peristalsis. When blood sugar spikes, the nerve fibres wither, acetylcholine release dwindles, and the entire gastric orchestra falls silent. The result? Early satiety, relentless bloating, and nausea that feels like the tide pulling you under. And heaven forbid you attempt a hearty meal – the sheer volume triggers a retro‑grade vomit that could embarrass a seasoned chef. The overlap with GERD and IBS isn’t a coincidence; it’s a symptom masquerade, a cruel trick of the body trying to signal distress in whatever language it can muster. Yet the medical community, armed with only endoscopic eyes, often mistakes the masquerade for a different villain, leading to misdiagnoses that squander months, if not years, of effective management. The truth lies in gastric emptying scintigraphy – a radioactive masterpiece that watches food’s journey like a time‑lapse movie, finally exposing the sluggish conveyor belt. But technology is evolving; wireless motility capsules now whisper real‑time pH, pressure, and temperature data, promising a future where we can catch the dysfunction before it derails daily life. Meanwhile, dietary strategies become a tactical operation – pureed foods, low‑fat, low‑fiber, eaten in bite‑sized symphonies, each morsel meticulously chewed to the size of a grain of rice. Prokinetics, like metoclopramide, march into the battlefield, but they’re a double‑edged sword, wielding the risk of tardive dyskinesia after twelve weeks. And let’s not forget the experimental pilots – gastric electrical stimulation devices, a sort of pacemaker for the gut, offering modest relief for chronic nausea. In short, diabetic gastroparesis is not a solitary ailment; it is a convergence of metabolic mayhem, neurological decay, and gastrointestinal rebellion, demanding a multidisciplinary assault that marries tight glycemic control with savvy gastro‑management. So, dear patients, arm yourselves with knowledge, demand comprehensive testing, and never settle for a vague “it’s probably GERD” diagnosis.
First off, kudos for tackling this topic – it can be overwhelming for folks just hearing about gastroparesis. A practical tip: keep a symptom diary, noting not only what you eat but also blood‑sugar spikes; patterns often emerge that help your doctor pinpoint the issue. Also, don’t shy away from asking for a wireless motility capsule – it’s less intimidating than the traditional scintigraphy and gives you a clear picture. Remember, small frequent meals are your friend, and staying hydrated can ease constipation that often co‑exists. Lastly, seek a dietitian experienced with diabetes; they’re gold when it comes to crafting low‑fat, low‑fiber menus that still taste good.
i think the post misses the point that most doc treat gastroparesis as just nausea. its more than that and you need a proper test not just symptoms. also the med list is too short many people cant get domperidone.
While I appreciate the thoroughness of the original article, one cannot overlook the fact that the therapeutic landscape is rapidly shifting. For instance, the emerging field of microbiome modulation suggests that probiotic regimens may, in fact, influence gastric motility indirectly by attenuating low‑grade inflammation. Moreover, the reliance on metoclopramide as a first‑line prokinetic is increasingly questioned given its neurological side‑effects, prompting many clinicians to consider low‑dose erythromycin cycles despite concerns about antibiotic resistance. It’s also worth noting that device‑based therapies, such as gastric electrical stimulation, have moved beyond experimental status in several tertiary centers, offering a salvage option for refractory cases. In practice, this means the management algorithm should be dynamic, integrating pharmacologic, dietary, and interventional strategies tailored to patient comorbidities and glycemic targets. Ultimately, a multidisciplinary approach-endocrinology, gastroenterology, nutrition, and even psychology-to address the quality‑of‑life impact can’t be overstated.
Great rundown! 😊 If you’re dealing with gastroparesis, consider trying the “chew‑thoroughly” rule – it really helps reduce that chunky feeling. Also, don’t forget to keep your appointments for gastric emptying studies; they’re the only way to be sure. Stay on top of blood sugar and you’ll see some improvement over time. 👍
Small meals, big difference.
The pathophysiological nexus between hyperglycemic autonomic neuropathy and delayed gastric emptying underscores a paradigmatic shift in our therapeutic algorithms. One must integrate both glycemic indices and motility metrics to formulate a truly evidence‑based regimen.
From an analytical perspective, the post could have emphasized the quantitative thresholds for HbA1c that correlate with gastroparesis incidence. In recent cohorts, an HbA1c >9% tripled the odds, which is a critical data point for clinicians to flag early.
Honestly, this stuff feels like a nightmare for anyone who actually enjoys food. You’re stuck watching your meals sit there like a bad plot twist. Whatever, I guess the meds help a bit, but the whole thing just sucks.
From a cultural standpoint, many traditional diets are high in fiber, which can exacerbate symptoms for gastroparesis patients. It’s worth exploring how regional cuisine adaptations can alleviate the burden while preserving heritage.
The statistical overlap between gastroparesis and IBS is not a coincidence; both involve dysregulated enteric nervous system signaling. Ignoring this shared mechanism leads to fragmented care and suboptimal outcomes.
Exactly! The whole multidisciplinary approach is key – diet, meds, and monitoring. 🌟 Remember, keeping a log of meals and blood sugars can be a game‑changer. 💪
Stay positive, friends! Even with gastroparesis, small steps – like sipping smoothies and keeping blood sugar steady – can make a huge difference. 🌈✨
Allow me to clarify: gastroparesis isn’t merely “slow stomach” – it’s a complex interplay of autonomic neuropathy, altered smooth‑muscle electrophysiology, and metabolic dysregulation. Neglecting any one facet leads to incomplete treatment.